

Location, location, location: fighting hematological malignancies by trapping T cells in lymphoid tissue
PRESS RELEASE
March 2022
Location, location, location: fighting hematological malignancies by trapping T cells in lymphoid tissue
Priothera’s small molecule mocravimod is transforming the prospects of patients with hematological malignancies receiving hematopoietic stem cell transplants.
In recent decades, the incidence of some hematologi- cal malignancies (HMs), such as acute lymphocytic leukemia (ALL) and chronic myeloid leukemia (CML), has decreased, while the incidence of others, includ- ing chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML), have increased in most countries worldwide. At the same time, the number of allogeneic hematopoietic stem cell transplants (HSCT), an established treatment for HMs following chemotherapy, has also increased significantly.
Yet HMs remain a major cause of cancer deaths, with overall mortality rising in recent decades—a fact that points to the unsatisfactory curative poten- tial of HSCT. Priothera is addressing this clear unmet medical need with the development of mocravimod, a small-molecule immune-modulating adjunctive and maintenance therapy for HMs that is initially being developed for AML with the first study in adult AML patients to be initiated in 2022.
For many intermediate and high-risk AML patients, as well as those with other HMs, chemotherapy fol- lowed by HSCT is the only potentially curative treat- ment option, but one that comes with a significant risk of serious and sometimes fatal graft-versus- host-disease (GvHD). HSCT is also limited in efficacy, due to immune-suppressive comedications that aim at controlling GvHD: 40% of post-HSCT AML patients relapse, with a dismal prognosis of 2-year survival less than 20%.
Mediating graft-versus-host disease
A crucial factor that determines the effectiveness of HSCT in AML is whether the patient develops graft-versus-leukemia, in which transplanted alloge- neic T cells attack and eliminate host leukemic cells (Fig. 1). Notably, the beneficial alloreactive T cells responsible for GvL are the same T cells that medi- ate GvHD. However, despite the fact that GvL and GvHD are driven by the same cells, they manifest in different parts of the body. GvL mainly occurs in lymphoid tissues, such as bone marrow and lymph nodes, where the malignant hematological cells reside. GvHD, by contrast, occurs throughout the body after donor T cells become activated by anti- gen-presenting cells and migrate out of lymphoid tissues to the gastrointestinal tract, liver, lung, and skin, where they attack host cells.
HSCT would be much more effective, and less prone to severe side effects, if alloreactive T cells remained in lymphoid tissues, mediating GvL but unable to attack the rest of the body to elicit GvHD.
This is mocravimod’s mode of action, which has been shown in a clinical proof-of-concept study. Mocravimod, a propane-1,3-diol derivative, is a novel, synthetic sphingosine 1-phosphate receptor (S1PR) agonist with a long half-life in the body. Delivered as a prodrug during and after HSCT, mocravimod is phosphorylated in vivo to generate the active moi- ety, mocravimod phosphate, which binds to S1PR, a receptor that plays a crucial role in lymphocyte trafficking from lymphoid tissues to peripheral blood.
By interfering with S1PR signalling, mocravimod sequesters donor T cells in lymphoid tissue, but with- out affecting their cytotoxic, leukaemia-cell-killing power, which facilitates the emergence of disease- eliminating GvL. At the same time, because the allore- active T cells cannot leave lymphoid tissues to attack other tissues within the body, the potential for GvHD is greatly reduced.
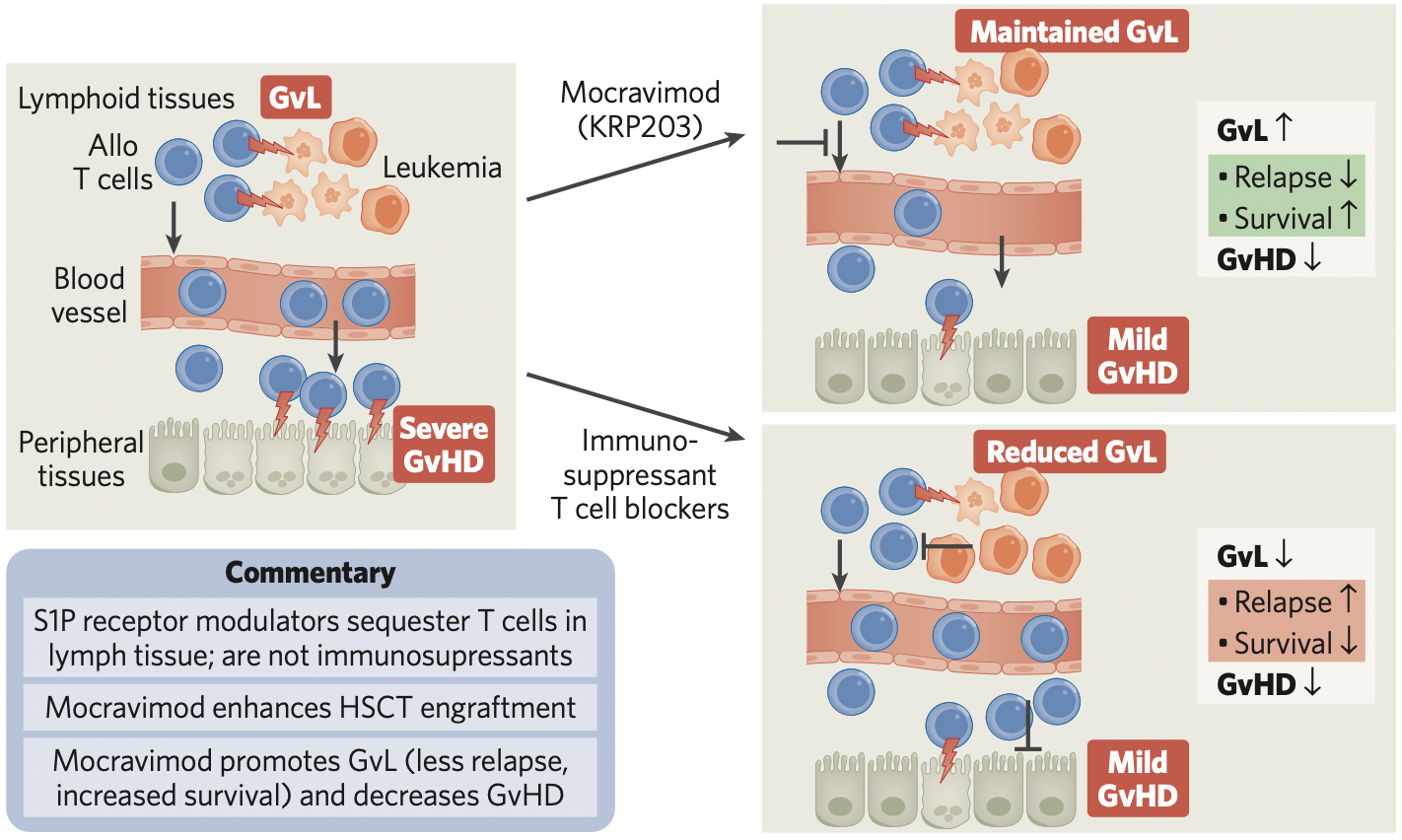
Fig. 1 | How mocravimod promotes GvL and hinders GvHD after HSCT. After hematopoietic stem cell transplant (HSCT), alloreactive T cells mediate beneficial graft-versus-leukemia (GvL) in lymphoid tissues and harmful graft-versus-host disease (GvHD) in peripheral tissue (left of figure). Mocravimod sequesters alloreactive T cells in lymphoid tissue, where they maintain GvL, and simultaneously reduce GvHD (top right). Other immunosuppressant T cell blockers can reduce GvHD, but also reduce GvL (bottom right).
Promising early clinical results
The dual effect has been demonstrated in mouse models of allogeneic HSCT, and mocravimod has been found to be safe and well-tolerated in human safety studies as well as in a study in which
hematologic malignancy patients requiring HSCT received mocravimod on top of standard of care. In these patients GvHD, relapse and mortality were reduced. In an upcoming phase 2b filing-enabling study, adult patients with AML will receive mocravi- mod as an adjunctive and maintenance therapy while undergoing HSCT. The study will assess improvements in relapse-free and overall survival.
Priothera has completed the necessary non-clinical filing package and is currently scaling up and validat- ing manufacturing processes for commercial supply so that the company will be immediately ready for its global commercial launch following approval. Priothera welcomes discussions with potential inves- tors and partners who are excited about ushering a new era of treatment for hematological malignancies.
Contact
Priothera
Philippe Lievre, Chief Business Officer Priothera
T: +41 76 802 11 30
E : philippe.lievre@priothera.com

Priothera – FDA and EMA Grant Orphan Drug Designation to mocravimod for the treatment of Acute Myeloid Leukemia (AML) in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT)
PRESS RELEASE
March 2022
Priothera – FDA and EMA Grant Orphan Drug Designation to mocravimod for the treatment of Acute Myeloid Leukemia (AML) in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT)
Mocravimod is being developed as a potential best-in-class adjunctive and maintenance therapy to enhance the curative potential of HSCT for AML patients.
A global registration-enabling Phase 2b trial assessing mocravimod in AML patients undergoing allogeneic HSCT is planned in H2 2022.
Dublin, Ireland – 3rd March 2022 – Priothera, a late-clinical stage biotechnology company pioneering the development of its S1P receptor modulator drug, mocravimod, today announced that the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have both granted orphan drug designation (ODD) to mocravimod for the treatment of Acute Myeloid Leukemia (AML) in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). EMA’s ODD follows a recommendation from the Committee for Orphan Medicinal Products (COMP).
Florent Gros, Co-Founder and CEO of Priothera, commented: “The orphan drug designations we received for mocravimod from both the FDA and EMA are important milestones towards addressing the urgent, unmet needs of AML patients. Allogenic stem cell transplantation is the only potentially curative approach for AML patients but has unacceptably high mortality rates with current treatments. We are looking forward to initiating the global Phase 2b clinical trial with mocravimod in multiple centers in the US, Europe and Asia in the coming months.”
Mocravimod, a sphingosine 1 phosphate (S1P) receptor modulator which has been previously tested in multiple autoimmune indications, is being developed to enhance the curative potential of HSCT. Moreover, it has shown a clinically relevant benefit in an early clinical study in patients with hematologic malignancies undergoing HSCT.
A multicenter Phase 2b study evaluating the efficacy and safety of mocravimod as an adjunctive and maintenance therapy to HSCT in adult AML patients is planned for the second half of 2022. The study will include approximately 250 patients in several countries in Europe, the US and Asia, upon approvals from respective health authorities.
Orphan drug designation is reserved for medicines treating rare, life-threatening or chronically debilitating diseases.
About mocravimod
Mocravimod (also known as KRP203), is a novel, synthetic, sphingosine 1-phosphate receptor (S1PR) modulator with a long duration in the body. Phase 1 and Phase 2 trials successfully assessed mocravimod for safety and tolerability in several autoimmune indications. Promising data from a Phase 1b/2a clinical study in patients with hematological malignancies led Priothera to further develop mocravimod for the treatment of blood cancers.
Mocravimod will be investigated in a Phase 2b/3 study as a potential treatment for patients with Acute Myeloid Leukemia (AML) receiving allogeneic hematopoietic stem cell transplantation (HSCT). Allogenic HSCT is the only potentially curative approach for AML patients, but current treatments have unacceptably high mortality and morbidity rates.
Priothera leverages S1PR’s unique mode of action to maintain anti-leukemia activity – graft-versus leukemia (GVL) – while reducing tissue damage resulting from graft-versus-host disease (GVHD), a consequence of allogenic HSCT. This novel treatment approach – the only S1PR modulator treating blood cancers – tackles a high unmet medical need and intends to add quality life to patients.
About Priothera
Priothera is leading the way in developing orally applied sphingosine 1 phosphate (S1P) receptor modulators for the treatment of hematological malignancies. S1P receptor modulators are known to largely reduce egress of T cells from lymphatic tissues and not being immunosuppressants, thereby allowing for inhibition of graft-versus-host-disease (GvHD) while maintaining graft-versus-leukemia benefits in patients receiving HSCT.
Priothera which was founded in 2020 by an experienced team of drug development experts is headquartered in Dublin, Ireland. The Company is backed by international founding investors Fountain Healthcare Partners (Dublin, Ireland), funds managed by Tekla Capital Management, LLC (Boston, Massachusetts), HealthCap (Stockholm, Sweden) and EarlyBird Venture Capital (Berlin, Germany).
For more information please visit: www.priothera.com
Contacts
Priothera
Florent Gros, CEO
E : info@priothera.com
MEDiSTRAVA Consulting
Sylvie Berrebi, Sandi Greenwood, Frazer Hall
E: priothera@medistrava.com
T: +44 (0)7714 306525

Priothera Enters Loan Agreement of €17.5 Million with the European Investment Bank
PRESS RELEASE
February 2022
Priothera Enters Loan Agreement of €17.5 Million with the European Investment Bank
Loan will further support the conduct of a global registration-enabling clinical trial with mocravimod for the treatment of Acute Myeloid Leukemia, and for expansion into additional cancer indications.
Dublin, Ireland – 23 February 2022 – Priothera, a late-clinical stage biotechnology company pioneering the development of its S1P receptor modulator drug, mocravimod, today announces that it has entered a Loan Agreement of €17.5 million with the European Investment Bank (EIB). This loan will further support a European, US and Asian registration-enabling clinical trial with mocravimod in Acute Myeloid Leukemia (AML) patients receiving hematopoietic stem cell transplant (HSCT).
The loan facility of €17.5 million is divided into two tranches, the first of which is €10 million and unconditional. Priothera will request payment of the first tranche in 2022 to expand the clinical development of mocravimod and prepare for its commercial drug supply. The second tranche of €7.5 million is available upon achievement of specific manufacturing, clinical and regulatory milestones and will be used to finance clinical validation in CAR-T cancer indications.
Florent Gros, Co-Founder and CEO of Priothera, comments: “We are very grateful for EIB’s support, a major and high quality European financial institution, as the Company is at an inflection point of its clinical development and potential commercialization path. This financing allows Priothera to extend its financial visibility to 2024, allowing us to complete a global registration-enabling clinical trial, as well as exploring other blood cancer indications for mocravimod. This new funding tool will help reinforce Priothera’s global lead in developing S1P receptor modulators in oncology.”
Christian Kettel Thomsen, Vice-President of the EIB, said: “The European Investment Bank Group supports development of innovative and pioneering treatments and medicine by leading biotech and medtech companies in Ireland and across Europe. Priothera’s new drugs offer an opportunity to revolutionise treatment of leukaemia and other cancers and the EIB is pleased to agree to €17.5 million of new financing to accelerate the clinical development and commercialization of mocravimod.”
About mocravimod
Mocravimod (also known as KRP203), a propane-1,3-diol derivative, is a novel, synthetic, sphingosine 1-phosphate receptor (S1PR) agonist with long duration in the body. Phase 1 and Phase 2 trials successfully assessed mocravimod for safety and tolerability in several autoimmune indications. Promising data from a Ph1b/Ph2a clinical study with patients with haematological malignancies led Priothera to further develop mocravimod for the treatment of blood cancers.
Mocravimod will be investigated in a Phase 2b/3 study as a potential treatment for patients with Acute Myeloid Leukemia (AML) receiving HSCT. Allogenic HSCT is the only potentially curative approach for AML patients but remains having unacceptably high mortality and morbidity rates with current treatments.
Priothera leverages S1PRs unique mode of action to maintain anti-leukaemia activity while reducing tissue damage resulting from graft-versus-host disease (GVHD), a consequence of HSCT. This novel treatment approach – the only S1PR modulator treating blood cancers – tackles a high unmet medical need that intends to add quality life to patients.
About Priothera
Priothera is leading the way in developing orally applied sphingosine 1 phosphate (S1P) receptor modulators for haematological malignancies. S1P receptor modulators are known to largely reduce egress of T cells from lymphatic tissues and not being immunosuppressants, thereby allowing for inhibition of graft-versus-host-disease (GvHD) while enhancing graft-versus-leukaemia benefits in patients receiving HSCT.
Headquartered in Dublin, Ireland, Priothera was founded in 2020 by an experienced team of drug development experts Drs. Florent Gros, Stephan Oehen, Dhaval Patel, Christoph Bucher, Simone Seiter, Philippe Lievre and Brice Suire.
Founding investors are Fountain Healthcare Partners (Dublin, Ireland), funds managed by Tekla Capital Management, LLC (Boston, Massachusetts), HealthCap (Stockholm, Sweden) and EarlyBird Venture Capital (Berlin, Germany).
For more information please visit: www.priothera.com
About the European Investment Bank (EIB)
The EIB is the European Union (EU) long-term financing institution and its shareholders are the 27 EU Member States. Its mission is to contribute to the integration, balanced development and economic and social cohesion of EU Member States. It borrows large volumes of funds from the capital markets and lends them with very favourable terms to support projects which contribute to the achievement of EU objectives. The EIB is working to put the EU at the forefront of the next wave of innovation, especially in the health sector. In response to the Covid-19 health crisis, the EIB has released € 6 billion for investments in the health sector to support medical infrastructure, additional research activities or other financing related to vaccines and treatments. As a European bank supporting the climate, the EIB is one of the main fund providers in the green transition towards a more low-carbon and sustainable growth model.
Contacts
Priothera
Florent Gros, CEO
E : info@priothera.com
MEDiSTRAVA Consulting
Sylvie Berrebi, David Dible, Sandi Greenwood, Frazer Hall
E: priothera@medistrava.com
T: +44 (0)7714 306525

Priothera Appoints Elisabeth Kueenburg M.D., as Chief Medical Officer
CMO Press release
November 25, 2021
Priothera Appoints Elisabeth Kueenburg M.D., as Chief Medical Officer
Dr. Kueenburg, former Clinical Development Lead at Celgene, a Bristol Myers Squibb Company, to advance clinical development of mocravimod in Acute Myeloid Leukemia patients undergoing allogeneic hematopoietic stem cell transplant.
“The breadth of knowledge Elisabeth has gained working at Celgene, alongside her extensive clinical experience, makes her a crucial addition to our team,” said Florent Gros, Co-Founder and CEO of Priothera. “We are delighted to welcome Elisabeth during this exciting time as we look to progress mocravimod, into a Phase 2b/3 study as a potential treatment for patients with Acute Myeloid Leukemia receiving hematopoietic stem cell transplantation. The study is expected to begin in 2022.”
“I am pleased to join Priothera at such an important stage of its development,” said Dr. Kueenburg. “Mocravimod has the potential to address the significant unmet need of AML patients undergoing HSCT. I look forward to guiding mocravimod and future programs into the clinic and making an important contribution to Priothera’s future success.”
Dr. Kueenburg brings significant drug development and medical affairs experience from her years at Celgene where she most recently served as Clinical Development Lead. At Celgene she developed deep clinical development and medical affairs expertise, providing strategic insight and overseeing the coordination of multiple clinical trials, in the area of hematology and specifically in multiple myeloma. Furthermore, Dr. Kueenburg has supported the successful global launch of Celgene’s Revlimid.
Prior to her numerous roles at Celgene, Dr. Kueenburg spent more than 15 years in clinical practice and academic research specializing in oncology and hematology.
Dr. Kueenburg gained her Doctor of Medicine from the University of Vienna in Austria.
(Photo of Dr. Kueenburg available on request)
About Priothera
Priothera is leading the way in developing orally applied sphingosine 1 phosphate (S1P) receptor modulators for hematological malignancies. S1P receptor modulators are known to largely reduce egress of T cells from lymphatic tissues and not being immunosuppressants, thereby allowing for inhibition of graft-versus-host-disease (GvHD) while enhancing graft-versus-leukemia benefits in patients receiving HSCT. Headquartered in Dublin, Ireland, Priothera was founded in 2020 by an experienced team of drug development and biotech experts.
Founding investors are Fountain Healthcare Partners (Dublin, Ireland), funds managed by Tekla Capital Management, LLC (Boston, Massachusetts), HealthCap (Stockholm, Sweden) and EarlyBird Venture Capital (Berlin, Germany).
For more information please visit: www.priothera.com
Contacts
Priothera
Florent Gros, CEO
E : info@priothera.com
MEDiSTRAVA Consulting
Sylvie Berrebi, Sandi Greenwood, Frazer Hall
E: priothera@medistrava.com
T: +44 (0)7714 306525

Recent Comments