
Priothera – Clinical Mocravimod Data in Hematological Malignancies Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplant (aHSCT) Presented at the 2022 European Hematology Association (EHA) Congress
NEWS
June 2022
Priothera – Clinical Mocravimod Data in Hematological Malignancies Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplant (aHSCT) Presented at the 2022 European Hematology Association (EHA) Congress
Phase 1b/2a data shows that mocravimod is safe and well tolerated
Dublin, Ireland – 13th June 2022 – Priothera, a late-clinical stage biotechnology company pioneering the development of its S1P receptor modulator compound, mocravimod, presented first clinical data on mocravimod (also known as KRP203) in hematological malignancies patients undergoing allogeneic hematopoietic stem cell transplant (HSCT) at the European Hematology Association (EHA) 2022 Congress, which was held in Vienna, Austria, June 9-12th, 2022.
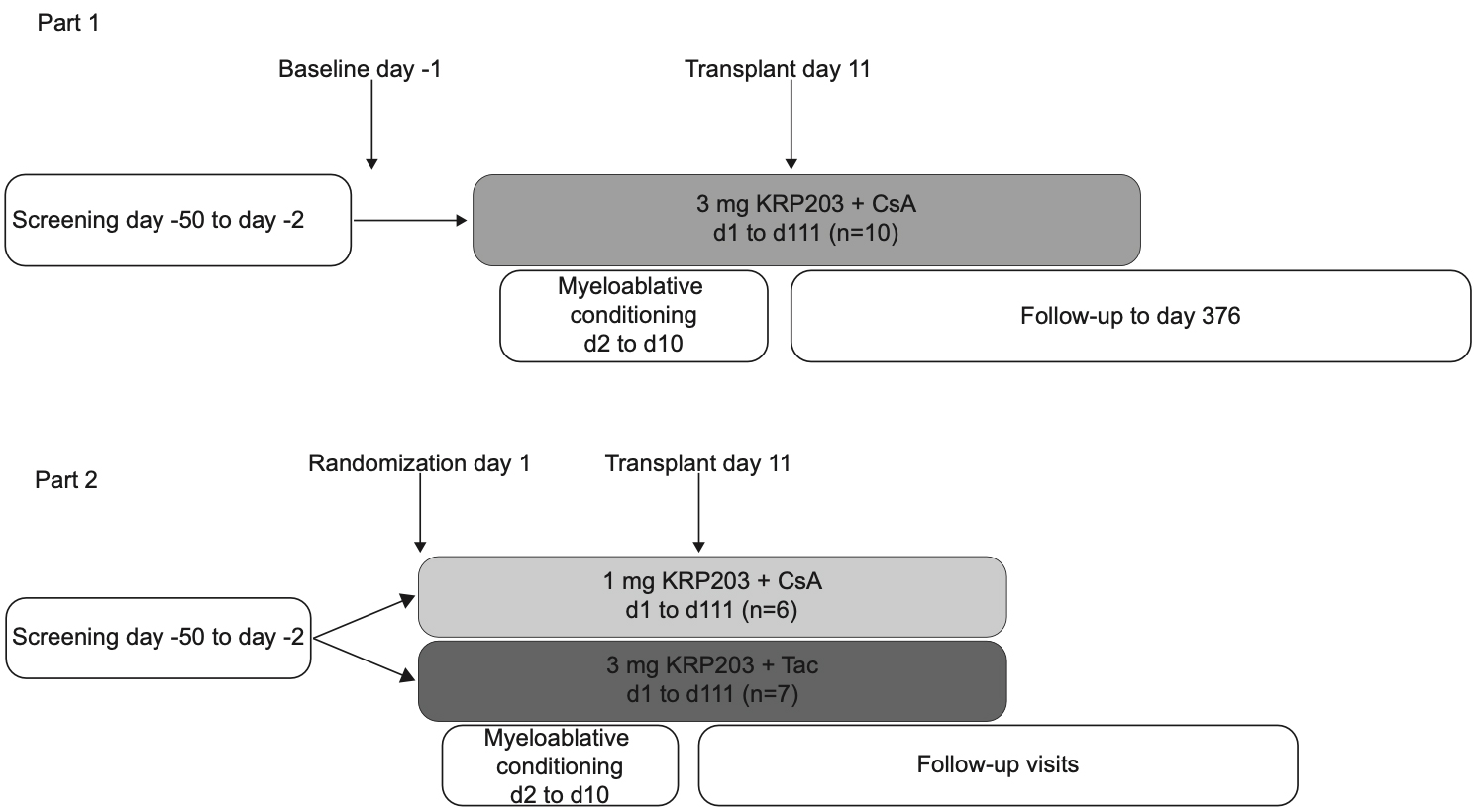
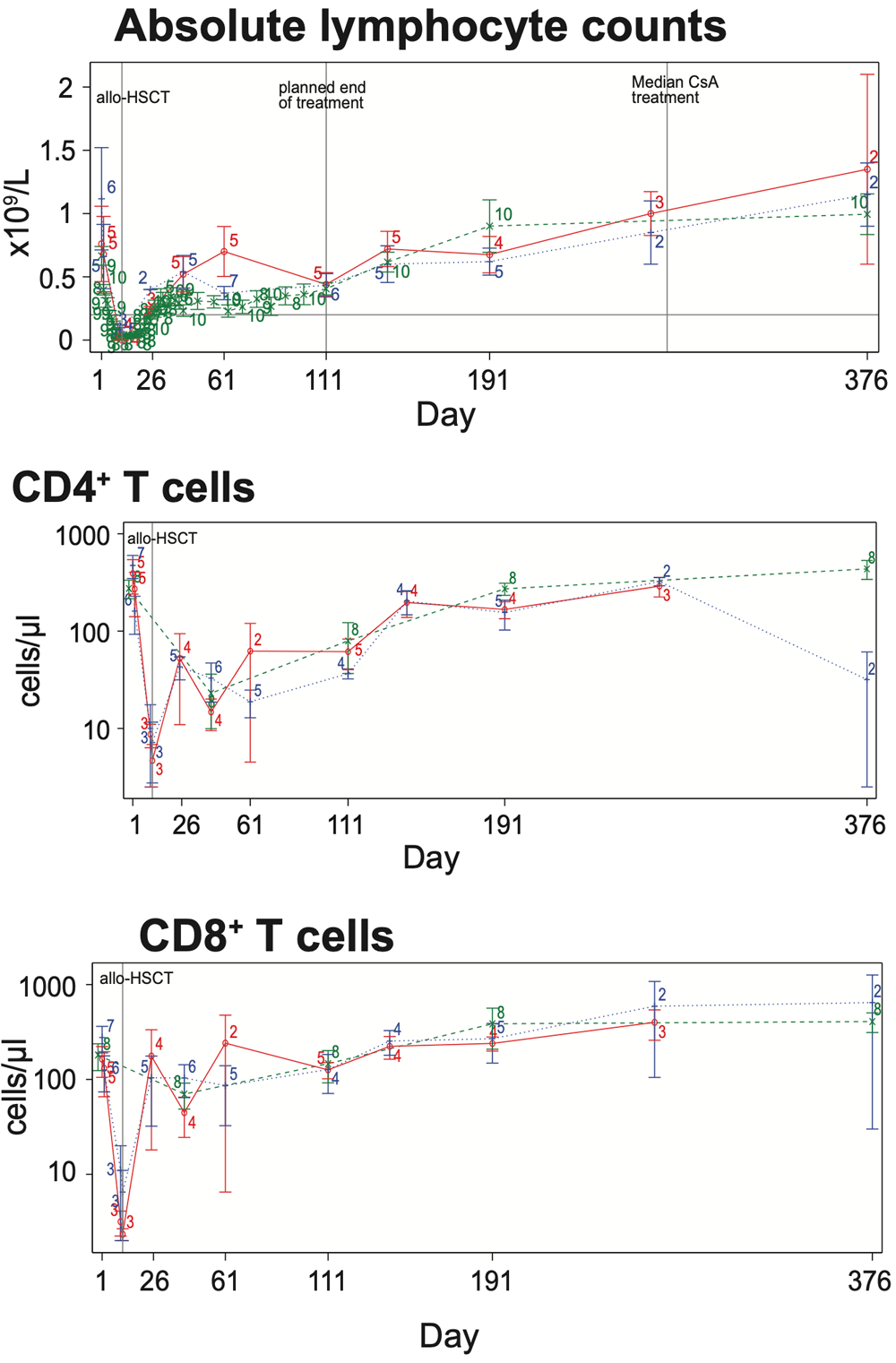
The oral presentation, which took place on 12th June, was entitled A two-part, single- and two-arm randomized, open-label study to evaluate the safety, tolerability and pharmacokinetics of KRP203 in subjects with hematological malignancies undergoing allogeneic hematopoietic stem cell transplantation. The study showed that in the Phase 1b/2a study mocravimod was safe and well tolerated with promising overall survival.
Key take aways from the data presentation were:
- The study provided first ever human data of a S1P receptor modulator administered to patients undergoing allogeneic HSCT
- S1PR modulator class effects, such as bradycardia, were of no clinical concern
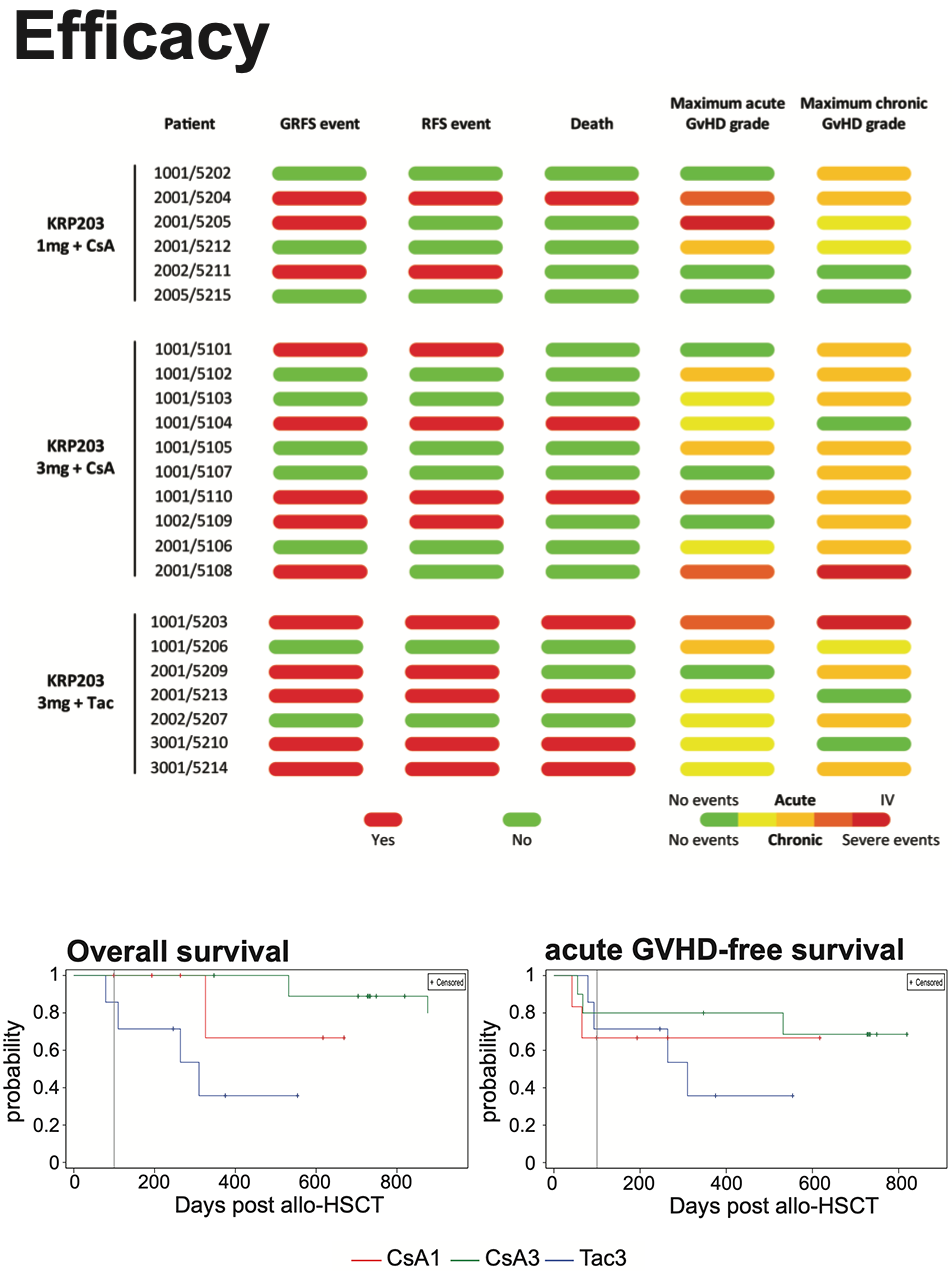
- Promising overall survival (OS) data
- Limited number of relapses, acute and chronic graft-versus-host disease (GVHD)
- Data supportive for conducting the planned MO-TRANS pivotal study to investigate efficacy (Relapse-free survival and OS) and GVHD in AML patients undergoing allogeneic HSCT
Priothera is initiating the pivotal MO-TRANS global Phase 2b/3 study in Europe, US and Japan, to assess the efficacy and safety of mocravimod as an adjunctive and maintenance therapy in adult AML patients undergoing allogeneic HSCT. The study is expected to start in the second half of 2022 and preliminary data from this study are expected by the end of 2024.
Florent Gros, Co-Founder and CEO of Priothera, said: “We are delighted to have been given the opportunity to present the full first human clinical data on mocravimod at the European Hematology Association Congress this year. Based on these results mocravimod has the potential to be a first-in-class therapy in maintaining graft-versus-leukemia responses while preventing graft-versus-host disease. This novel approach would provide a tremendous benefit to AML patients undergoing an allogeneic hematopoietic stem cell transplant. We are excited about mocravimod which leverages a well-described mode of action in a hematology/oncology setting and has been successfully used in autoimmune indications. Pre-clinical and clinical proof of concept studies have herewith demonstrated mocravimod’s ability to improve survival outcomes for this devastating disease. We look forward to advancing the global pivotal Phase 2/3 trial which is due to start in the second half of 2022.”
Priothera’s abstract is available on EHA2022 Congress Abstract Book, here: https://journals.lww.com/hemasphere/Documents/EHA2022%20Congress%20Abstract%20Book.pdf
About Mocravimod
Mocravimod (also known as KRP203) is a synthetic, sphingosine 1-phosphate receptor (S1PR) modulator. This novel investigational drug has been assessed in Phase 1 and Phase 2 trials for safety and tolerability, as well as for efficacy in several autoimmune indications. Promising data from a Phase 1b/2a clinical study in patients with hematological malignancies led Priothera to further develop mocravimod for the treatment of blood cancers and the improvement of CART cell therapy.
Mocravimod will be investigated as an adjunctive and maintenance treatment in a Phase 2b/3 study as a potential treatment for patients with Acute Myeloid Leukemia (AML) receiving allogeneic hematopoietic stem cell transplantation (HSCT). Allogeneic HSCT is the only potentially curative approach for AML patients, but current treatments have unacceptably high mortality and morbidity rates.
Priothera leverages S1PR modulator’s unique mode of action to maintain anti-leukemia activity – graft-versus leukemia (GVL) while reducing tissue damage resulting from graft-versus-host disease (GVHD), a consequence of allogeneic HSCT. This novel treatment approach – mocravimod being the only S1PR modulator treating blood cancers – tackles a high unmet medical need and intends to add quality life to patients.
About Priothera
Priothera is leading the way in developing orally applied sphingosine-1-phosphate (S1P) receptor modulators for the treatment of hematological malignancies and for the improvement of CART cell therapies. S1P receptor modulators are known to largely reduce egress of T cells from lymphatic tissues. Not being an immunosuppressant, mocravimod maintains the graft-versus-leukemia (GVL) benefits in patients receiving HSCT while inhibiting graft-versus-host-disease (GVHD).
Priothera was founded in 2020 by an experienced team of drug development experts and is headquartered in Dublin, Ireland, and with a subsidiary in Saint-Louis, France. The Company is backed by international founding investors Fountain Healthcare Partners (Dublin, Ireland), funds managed by Tekla Capital Management, LLC (Boston, Massachusetts), HealthCap (Stockholm, Sweden) and EarlyBird Venture Capital (Berlin, Germany).
For more information please visit: www.priothera.com or follow Priothera on LinkedIn www.linkedin.com/company/priothera/
Contact
Priothera
Florent Gros, CEO
E: info@priothera.com
MEDiSTRAVA Consulting
Sylvie Berrebi, Sandi Greenwood, Frazer Hall
E: priothera@medistrava.com
T: +44 (0) 203 928 6900



Recent Comments