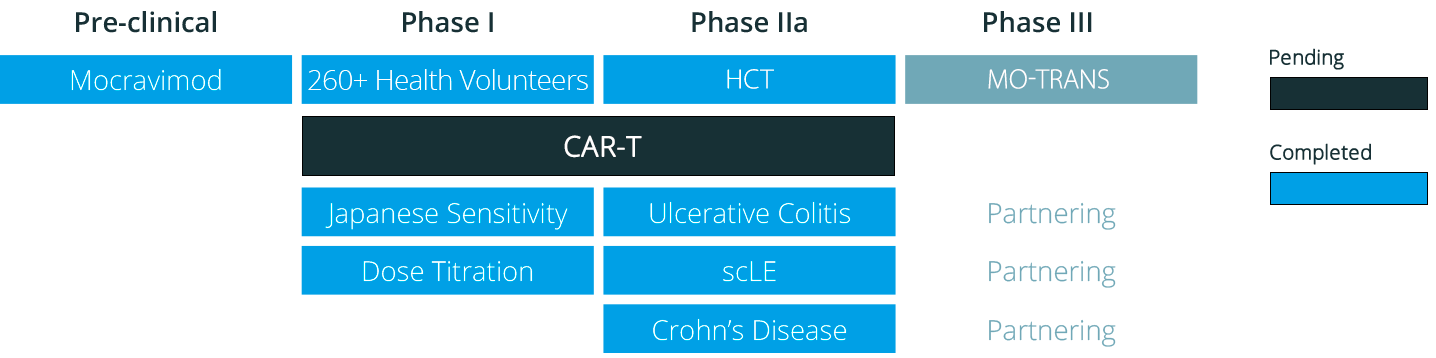
Our Pipeline
Our first-in-class clinical-stage asset mocravimod has entered a registration-enabling Phase 3.
In addition, we plan to investigate mocravimod in a CAR-T exploratory clinical study.
Mocravimod was tested in over 400 human subjects, including extensive Phase 1 studies, Phase 2a studies in autoimmune indications (Ulcerative Colitis, subacute cutaneous Lupus Erythematosus and Crohn’s Disease) and in a Phase Ib/IIa Hematopoietic Cell Transplant (HCT) study to treat hematological malignancies.