
Priothera to present an abstract at the TANDEM Meetings / Transplantation & Cellular Therapy Meetings of ASTCT™ and CIBMTR – April 23-26 2022
PRESS RELEASE
April 2022
Priothera to present an abstract at the TANDEM Meetings / Transplantation & Cellular Therapy Meetings of ASTCT™ and CIBMTR – April 23-26 2022
Simone Dertschnig1, Stephan Oehen, Dominik Heim, Jürgen Finke and Christoph Bucher.
Priothera SAS, St. Louis, France, 2University Hospital Basel, Basel, Switzerland, 3University of Freiburg, Freiburg, Germany.
Background
The success of allogeneic hematopoietic stem cell transplantation (HSCT) is limited by disease relapse. Alloreactive donor T cells have the potential to reject residual malignant cells and are essential to prevent relapse by graft-versus-leukemia (GVL) effect. This GVL response is very powerful, however, the same T cells cause acute graft-versus-host disease (aGVHD). T cell trafficking from lymphoid organs is a major step in the development of aGVHD and is regulated by sphingosine-1-phosphate (S1P) gradients. Pre-clinical data showed that pharmacological modulation of S1P receptor (S1PR) signaling by KRP203 sequesters T cells in lymphoid organs and prevents their egress to aGVHD target sites. In murine models, KRP203 efficiently reduced aGVHD without suppressing T cell function, while GVL was maintained and survival improved. Here, we show data of the first clinical trial investigating S1PR modulation by KRP203 as GVHD prophylaxis while maintaining GVL in patients undergoing HSCT for the tratement of hematological malignancies.
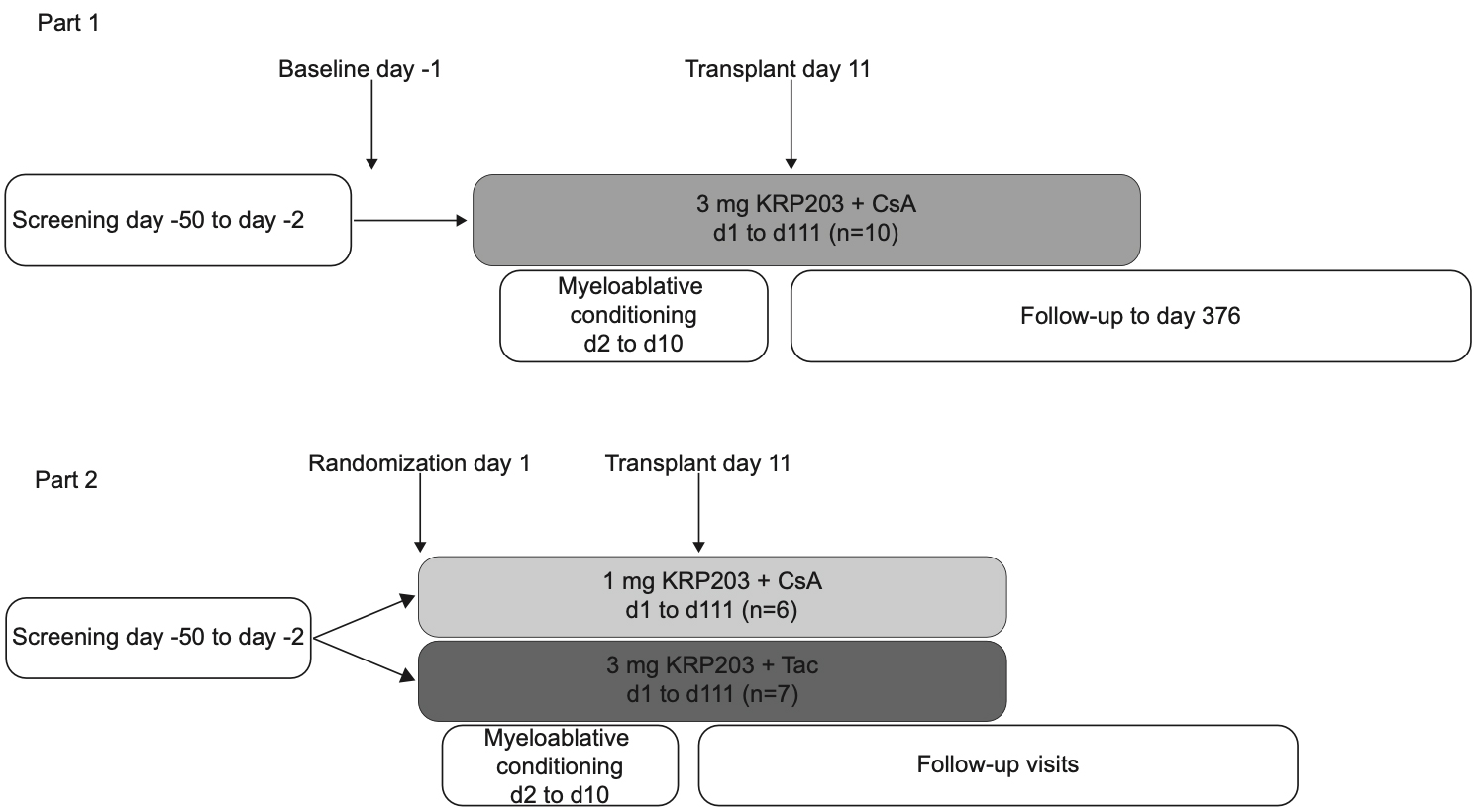
Study design
This multicentric, phase Ib, prospective, open label, two-part study evaluated safety, tolerability and pharmacokinetics of KRP203 in intermediate to high-risk patients undergoing allogeneic HSCT for hematological malignancies. Three treatment arms were investigated: 3 mg KRP203 + cyclosporine A (CsA)/methotrexate (MTX) (CsA3), 1 mg KRP203 + CsA/MTX (CsA1) and 3 mg KRP203 + tacrolimus (Tac)/MTX (Tac3). KRP203 was administered daily from day 1 until day 111 and HSCT was performed on day 11.
Results
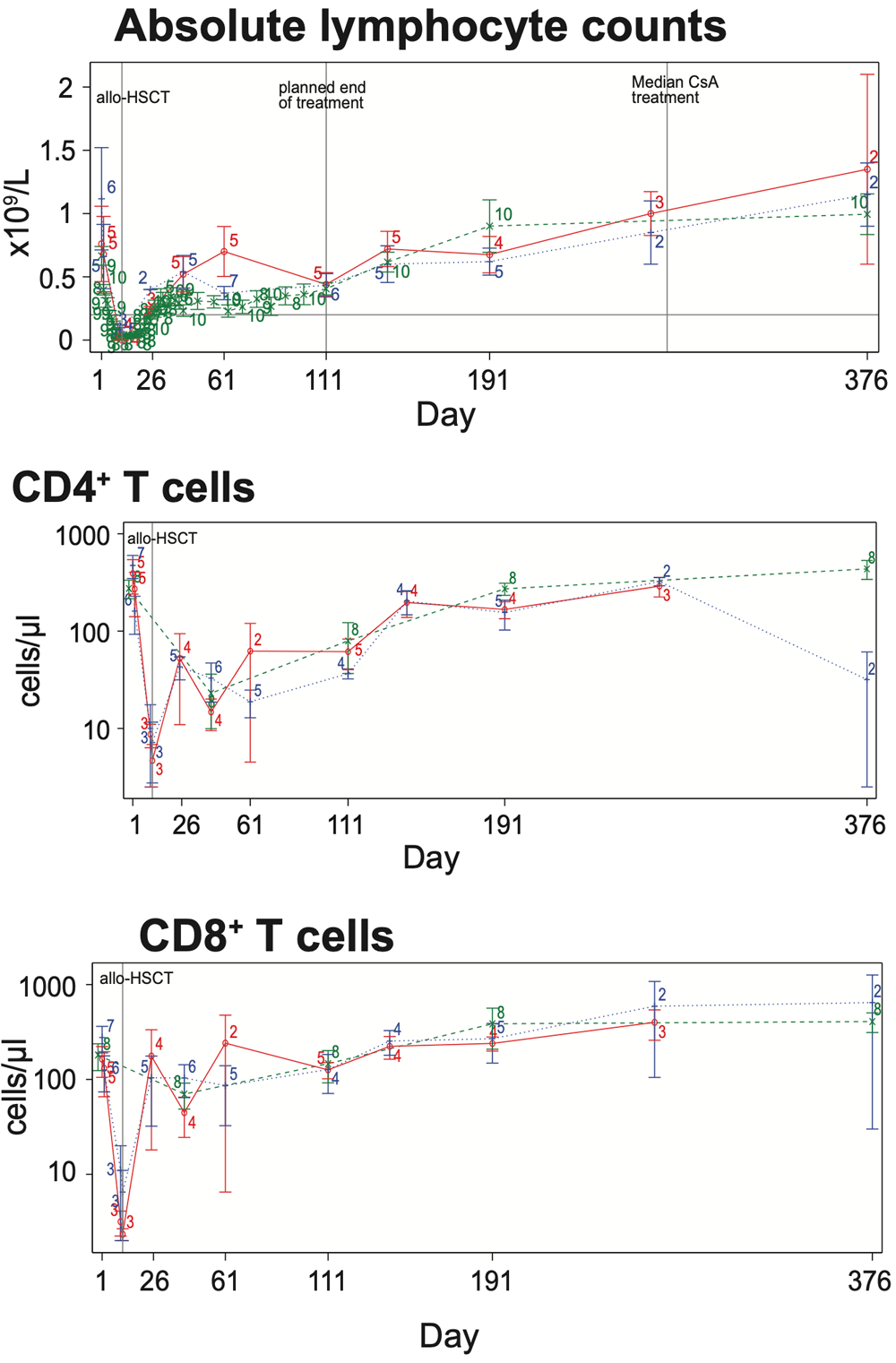
Upon KRP203 treatment, absolute lymphocyte counts (ALC) stabilized above 0.2×109/L but were efficiently reduced in all three treatment arms below pre-HSCT level (<0.7×109/L). Tretment effect was resolved when KRP203 was stopped on day 111, which caused an increase in blood ALC to pre-HSCT level. CD4+ and CD8+ T cells, GVL mediators and major players in the pathophysiology of aGVHD, were reduced in peripheral blood in response to KRP203 treatment. Only CD4+ T cells remained below pre-HSCT level (<100 cells/μl).
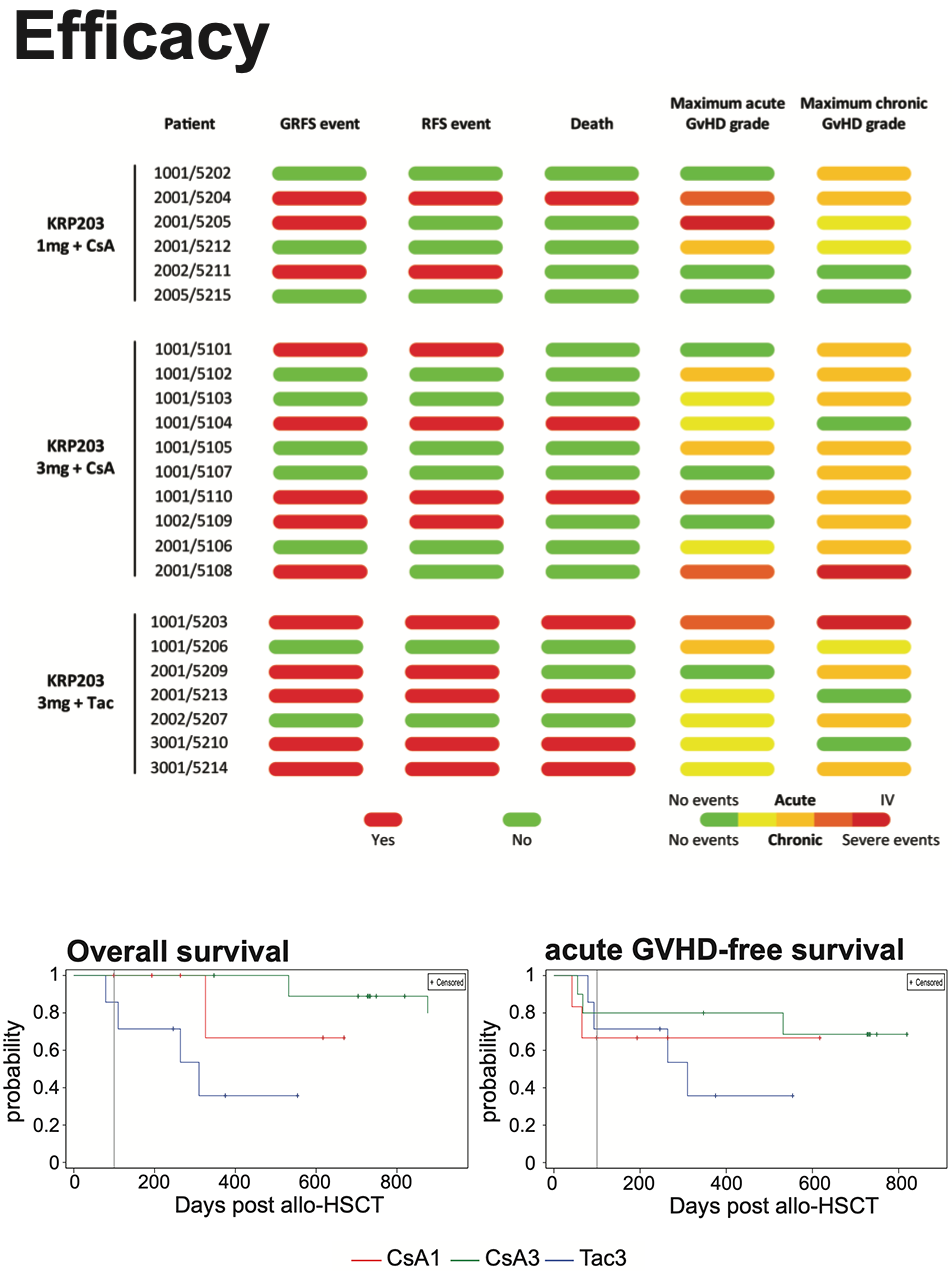
Overall survival probaility was highest for subjects in the CsA3 arm with the first death reported on day 532. Overall survival at 1 year was 100% in the CsA3 arm, 67% in the CsA1 arm and 36% in the Tac3 arm. The median time to relapse was 749 days. 2/6 subjects in the CsA1 arm, 4/10 subjects in the CsA3 arm and 4/7 subjects in the Tac3 arm reported relapse. The median time to any aGVHD was 55 days and 21.7% of subjects presented with grade III-IV aGVHD. The median time to any chronic GVHD was 174 das and 56.5% of subjects presented with moderate and 8.7% with severe chronic GVHD. 52.2% of subjects did not experience a GRFS event and were alive at 6 months.
Conclusion
KRP203 shows promising early clinical outcome with a limited number of relapses, acute and chronic GVHD. The results of this small study support the further investigations of mocravimod in a homogeneous patient population undergoing allogeneic HSCT to confirm safety and assess clinical efficacy, such as overall survival, relapse-free survival and GVHD-free survival.
TANDEM Meetings / Transplantation & Cellular Therapy Meetings of ASTCT™ and CIBMTR



Recent Comments