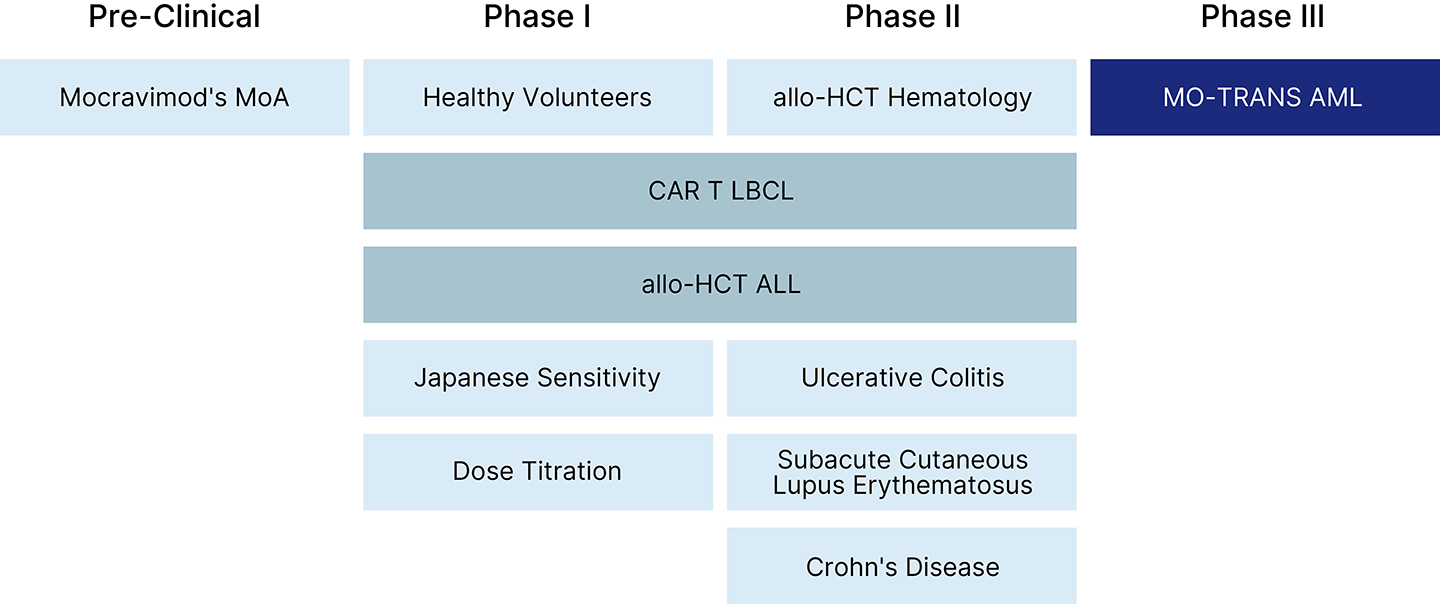
Our Pipeline
At Priothera, we are relentless in our pursuit of therapies that will help patients around the world.

Transforming Cancer Care Through Innovation and Commitment
Priothera is building a pipeline to enable the discovery, development and delivery of innovative medicines to patients with cancer. Our first-in-class clinical-stage asset, mocravimod, is currently undergoing a registration-enabling global Phase III clinical trial.
We are also planning to investigate the utility of mocravimod in other settings and disease states including the use with chimeric antigen recepter (CAR) T cells and possibly in other leukemias, including acute lymphocytic leukemia (ALL), one of the most devastating and common types of cancer in children.
In addition to patients enrolled in the Phase III clinical trial, mocravimod has been tested in over 400 human subjects, including extensive Phase I studies, Phase IIa studies in autoimmune indications (ulcerative colitis, subacute cutaneous lupus erythematosus and Crohn’s disease) and in a Phase Ib/IIa hematopoietic cell transplant (HCT) study to treat hematological malignancies.